ANALYTICAL CHEMISTRY -COLLINS KEMBOI
SCHEME 968 RESENTS A NOTE ON ERROR : CONTENT, STYLES, WORK, FONTS: VALUE CALCULATED CAN BE DETERMINE AS BELOW:
Errors
Error can be defined as numerical difference between observed value and true value.[15]
In error the true value and observed value in chemical analysis can be related with each other by the equation
where
- is the absolute error.
- is the true value.
- is the observed value.
Error of a measurement is an inverse measure of accurate measurement i.e. smaller the error greater the accuracy of the measurement.
Errors can be expressed relatively. Given the relative error():
The percent error can also be calculated:
If we want to use these values in a function, we may also want to calculate the error of the function. Let be a function with variables. Therefore, the propagation of uncertainty must be calculated in order to know the error in :
Standards
Standard curve
A general method for analysis of concentration involves the creation of a calibration curve. This allows for determination of the amount of a chemical in a material by comparing the results of unknown sample to those of a series of known standards. If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method a known quantity of the element or compound under study is added, and the difference between the concentration added, and the concentration observed is the amount actually in the sample.
Internal standards
Sometimes an internal standard is added at a known concentration directly to an analytical sample to aid in quantitation. The amount of analyte present is then determined relative to the internal standard as a calibrant. An ideal internal standard is isotopically-enriched analyte which gives rise to the method of isotope dilution.
Standard addition
The method of standard addition is used in instrumental analysis to determine concentration of a substance (analyte) in an unknown sample by comparison to a set of samples of known concentration, similar to using a calibration curve. Standard addition can be applied to most analytical techniques and is used instead of a calibration curve to solve the matrix effect problem.
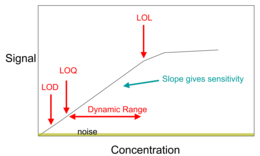
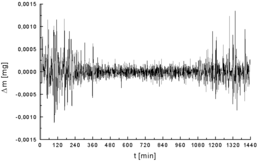
Signals and noise[
One of the most important components of analytical chemistry is maximizing the desired signal while minimizing the associated noise.[16] The analytical figure of merit is known as the signal-to-noise ratio (S/N or SNR).
Noise can arise from environmental factors as well as from fundamental physical processes.
Thermal noise
Thermal noise results from the motion of charge carriers (usually electrons) in an electrical circuit generated by their thermal motion. Thermal noise is white noise meaning that the power spectral density is constant throughout the frequency spectrum.
The root mean square value of the thermal noise in a resistor is given by[16]
where kB is Boltzmann's constant, T is the temperature, R is the resistance, and is the bandwidth of the frequency .
Shot noise
Shot noise is a type of electronic noise that occurs when the finite number of particles (such as electrons in an electronic circuit or photons in an optical device) is small enough to give rise to statistical fluctuations in a signal.
Shot noise is a Poisson process and the charge carriers that make up the current follow a Poisson distribution. The root mean square current fluctuation is given by[16]
where e is the elementary charge and I is the average current. Shot noise is white noise.
Flicker noise
Flicker noise is electronic noise with a 1/ƒ frequency spectrum; as f increases, the noise decreases. Flicker noise arises from a variety of sources, such as impurities in a conductive channel, generation and recombination noise in a transistor due to base current, and so on. This noise can be avoided by modulation of the signal at a higher frequency, for example through the use of a lock-in amplifier.
Environmental noise
Environmental noise arises from the surroundings of the analytical instrument. Sources of electromagnetic noise are power lines, radio and television stations, wireless devices, Compact fluorescent lamps[17] and electric motors. Many of these noise sources are narrow bandwidth and therefore can be avoided. Temperature and vibration isolation may be required for some instruments.
Noise reduction
Noise reduction can be accomplished either in computer hardware or software. Examples of hardware noise reduction are the use of shielded cable, analog filtering, and signal modulation. Examples of software noise reduction are digital filtering, ensemble average, boxcar average, and correlation methods.[16]
Applications
 Analytical chemistry has applications including in forensic science, bioanalysis, clinical analysis, environmental analysis, and materials analysis. Analytical chemistry research is largely driven by performance (sensitivity, detection limit, selectivity, robustness, dynamic range, linear range, accuracy, precision, and speed), and cost (purchase, operation, training, time, and space). Among the main branches of contemporary analytical atomic spectrometry, the most widespread and universal are optical and mass spectrometry.[18] In the direct elemental analysis of solid samples, the new leaders are laser-induced breakdown and laser ablation mass spectrometry, and the related techniques with transfer of the laser ablation products into inductively coupled plasma. Advances in design of diode lasers and optical parametric oscillators promote developments in fluorescence and ionization spectrometry and also in absorption techniques where uses of optical cavities for increased effective absorption pathlength are expected to expand. The use of plasma- and laser-based methods is increasing. An interest towards absolute (standardless) analysis has revived, particularly in emission spectrometry.
Analytical chemistry has applications including in forensic science, bioanalysis, clinical analysis, environmental analysis, and materials analysis. Analytical chemistry research is largely driven by performance (sensitivity, detection limit, selectivity, robustness, dynamic range, linear range, accuracy, precision, and speed), and cost (purchase, operation, training, time, and space). Among the main branches of contemporary analytical atomic spectrometry, the most widespread and universal are optical and mass spectrometry.[18] In the direct elemental analysis of solid samples, the new leaders are laser-induced breakdown and laser ablation mass spectrometry, and the related techniques with transfer of the laser ablation products into inductively coupled plasma. Advances in design of diode lasers and optical parametric oscillators promote developments in fluorescence and ionization spectrometry and also in absorption techniques where uses of optical cavities for increased effective absorption pathlength are expected to expand. The use of plasma- and laser-based methods is increasing. An interest towards absolute (standardless) analysis has revived, particularly in emission spectrometry.
[citation needed]- BEST ANALYTICAL CHEMISTRY BOOK IN THE WORLD (ZUMDAL GENERAL CHEMISTRY TERTIARY BOOK)















Comments